Pupfish are indeed the only group of fish named after puppy dogs for their playful behavior. They’re best known for their ability to survive in extreme environments, like desert hot springs. However, for my dissertation research, I have focused on understanding their evolution and diversification.
Pupfish show a remarkable pattern of adaptive diversification: in only two small lake systems throughout their entire range, pupfishes are evolving from 50 – 130 times faster than all other pupfish species. Truly ‘explosive evolution‘ – the fastest morphological diversification rates measured so far in fishes, and one of the fastest rates documented among all organisms. Further, other pupfish groups of similar young age do not show such extreme rates.
Figure 3 in paper. The pupfish heat map. Colors indicate the rate of evolution for 16 traits relative to other pupfishes in a: Lake Chichancanab pupfishes and b: San Salvador Island pupfishes.
What is going on here? The short answer is the evolution of novel ecological niches. Cyprinodon pupfishes occur throughout the Caribbean and along the Atlantic coast from Massachusetts to Venezuela and as far inland as isolated springs in California and Mexico. Throughout their entire range, pupfishes are ecological generalists: they eat mostly algae, decaying vegetation, and whatever insects or crustaceans they can catch. Yumm! Although different species can often be distinguished by differences in male coloration, or subtle differences in body or fin shape, pupfish species on the whole are anatomically very similar, particularly in jaw shape. Further, multiple pupfish species never coexist in the same habitat.
Except in two places. These are the only two places throughout their entire range where multiple pupfish species coexist and specialize on entirely new resources. On the tiny island of San Salvador in the Bahamas (only 11 miles long!), three pupfish species coexist in the inland salty lakes. Incredibly, one of these has evolved to feed almost entirely on the scales of other pupfishes! While scale-eating has evolved at least 14 times in other groups of fishes, within the 1,500 species of atherinimorphs, to which pupfish belong, this undescribed pupfish species is the only known scale-eater! While previous researchers speculated that it may eat scales or other fish, I was stunned to find only scales and no whole fish when I began examining the guts of this species (n = 60). This behavior is easy to watch in the field – the scale-eater stalks any nearby pupfish, quickly orienting perpendicular to its prey, striking and biting off scales, then stealthily moving on to the next target, just like a pup-tiger.
Cyprinodon sp. ‘scale-eater’: Males in full breeding coloration photographed in their natural habitat on San Salvador Island.
There is a second ecologically specialized species in these San Salvador lakes. This species has shortened jaws for crushing its diet of snails and ostracods. Moreover, it has a nose! This is one of the few fish species that tucks its jaw underneath protruding nasal tissue surrounding protruding bones (maxilla and nasal) on the face of the fish.
Cyprinodon sp. ‘nose’ What looks like an upper lip in this photo is actually the fish’s nose protruding outward above the fish’s tucked upper jaw.
The function of this peculiar fish nose is so far unknown (or any fish nose, for that matter). I do have a couple guesses: perhaps it helps stabilize the fish’s jaw while crushing hard shells. Or, it may help with species recognition, as males gently nudge females when trying to entice them to spawn.
The second remarkable place for pupfish diversification is Lake Chichancanab, Mexico, a large, brackish lake in the center of the Yucatan peninsula (Chichancanab is Mayan for “little lake” or “little girl lake”, whichever you prefer). Chichancanab contained at least five coexisting species of pupfishes, including four ecological specialists. One of these, Cyprinodon maya, is the largest pupfish species known and also the only pupfish to eat other fish. A second species, Cyprinodon simus, is the second smallest pupfish species, and was observed feeding on zooplankton in large shoals in open water. Piscivory and zooplanktivory are unique pupfish niches found only in Lake Chichancanab.
Terribly, these descriptions of Chichancanab species are in past tense. In the early 1990’s, invasive African tilapia (probably Oreochromis mossambicus) were introduced to Lake Chichancanab. In addition, the native Mexican tetra, Astyanax sp., was also introduced. All specialized pupfish species promptly declined in abundance and frequency over the next 10 years. I visited the lake in 2009 and after surveying thousands and thousands of fish from several different basins of the large lake, I observed zero Cyprinodon maya and only one putative hybrid Cyprinodon simus. These specialized species are now functionally extinct in the lake. Thankfully, they have survived in home aquaria and backyard fish ponds in the US thanks to the efforts of dedicated aquarium hobbyists in the American Killifish Association. I am now maintaining these extinct-in-the-wild species in the lab as well.
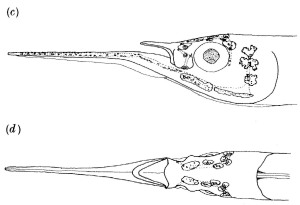
Cleared and stained specimenof Cyprinodon maya (top), the only piscivore pupfish.
Cleared and stained specimen of Cyprinodon simus (bottom), the only zooplanktivore pupfish. Note the dramatic difference in the thickness of their lower and upper jaws. These specimens were collected in the wild before invasive species were introduced and generously loaned for this research by the University of Michigan Museum of Zoology.
Thus, in only two remarkable lake systems throughout their entire range, pupfish are speciating and adapting to novel trophic resources, like scales, snails, other fish, and plankton. These two groups of pupfishes also happen to be showing the fastest rates of evolution among all pupfishes. Probably not a coincidence: invasion of these novel ecological niches is driving incredible rates of morphological change, particularly in jaw shape.
It is particularly remarkable to see this pattern within pupfish, a group of fishes that has repeatedly been isolated in new, extreme environments and also probably has repeatedly adapted to these new environments. Several other groups of pupfishes were also evolving fast in my analysis – around 5 – 10 times faster than average, such as the groups containing the Devil’s Hole pupfish, a tiny species restricted to the smallest habitat of any known organism, a tiny cave shaft in Death Valley, shown here:
Devil’s Hole, Death Valley National Park, Nevada. This vertical shaft of water stays a balmy 94 degrees F year-round and divers have not yet found the bottom (at least 400 feet deep). Cyprinodon diabolis is restricted to eating scarce algae off a tiny rock shelf near the surface and its population size has fluctuated between 37 and around 400 fish.
Cyprinodon pachycephalus also belongs to a quickly evolving group. This is the pupfish species that lives and breeds in the hottest waters of any known vertebrate, 114 degrees Fahrenheit year-round!
These are incredibly extreme environments that would be expected to drive rapid rates of morphological evolution. Indeed, these species are changing quickly, but the Devil’s hole pupfish and C. pachycephalus are both generalist detritivores, just like their relatives.
However, to really see explosive evolution appears to require that pupfish start dabbling in entirely new ways of life, to go where no pupfish has ever gone before. (this wouldn’t be blogging without Star Trek!)
But, I haven’t yet fully answered the question I originally posed. Why have novel trophic niches evolved in these two places and nowhere else across their entire range? Certainly, the size of these two lakes and lack of competitors (except native mosquitofishes) plays a role. But, there may be many similar lakes with similar fish communities throughout the Caribbean. What is going on here? This remains an outstanding research question, one I am actively pursuing.
For the full story and contact information, please see the paper:













Recent Comments